Warning after reports of reactions to Botox-like product
f
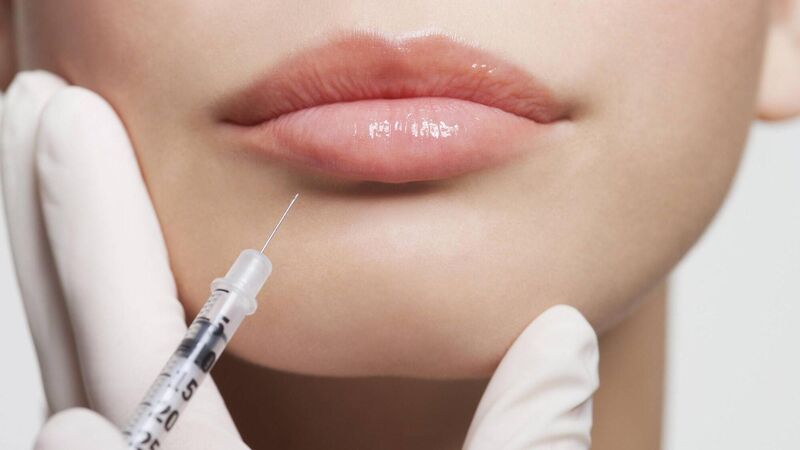
For those who swear by it, to those who swear against it, Botox is a controversial topic.
Many celebrities who face so much pressure to look flawless see it as an opportunity to keep facial wrinkles and lines at bay.
Pamela Anderson, Cindy Crawford, Demi Moore, and Brooke Shields all admit to using Botox injections. Crawford has gone as far as saying: “I owe the quality of my skin to my cosmetic surgeon.”
However, risks attached to the use of unlicensed products in unqualified hands are increasingly apparent.
The Irish health products watchdog is reported to be closely monitoring the potential use of unlicensed Botox-like products after health officials in the UK were notified of dozens of people suffering adverse reactions after receiving cosmetic procedures involving botulinum toxin.
This recent event heightens concerns around the significant risk to individuals who are receiving cosmetic procedures involving unlicensed products, both in Ireland and further afield.
Botulinum toxin is a prescription-only medicine. Buying the product in any other circumstances significantly increases the risk of getting a product that is either falsified or not licensed for use in Ireland. This means there are no safeguards to ensure products meet the regulated standards for quality and safety.
In mid-July, the UK Health Security Agency (UKSHA) warned the public to be aware of the signs and symptoms of botulism after a number of people presented to NHS healthcare settings following adverse reactions after receiving cosmetic procedures involving botulinum toxin.
At that time, it said 28 cases of iatrogenic botulism were reported to British health authorities between July 4 and 14. They presented with a mix of symptoms, including difficulty swallowing, slurred speech and breathing difficulties that required respiratory support.
The latest update shows the number of reported cases now exceeds 40.
The Irish Health Products Regulatory Authority (HPRA) said in a recent statement that while it has not been notified of similar cases here, it is liaising with UK officials to assess any potential risk to the Irish public.
“While UK investigations remain ongoing, the evidence to date suggests these cases are associated with the use of an unlicensed Botox-like product,” it said.
The UKHSA said it was understood that the UK-based practitioners involved in administering the unlicensed product have ceased providing the procedure and are co-operating with the ongoing investigation.
The HPRA said that “at this time, there is no evidence of this issue impacting the Irish market and, to date, the HPRA has not been notified of any similar reports of adverse reactions occurring in Ireland”.
The HPRA advised any member of the public who has been treated with botulinum toxin, including those doing so outside of a health service setting, to contact their doctor and seek medical attention immediately if they experience difficulties breathing.
In relation to the recent UK cases, Co-National Medical Director of secondary care at NHS England Professor Meghana Pandit, did not mince her words: “When these procedures go wrong, there is a risk of serious infections and permanent scarring, which is why only registered professionals should be prescribing these treatments.”
The number of products that have been detained by the HPRA, purporting to contain botulinum toxin as part of a range of illegal medicines, has skyrocketed in recent years.
It is reported that last year, 1,709 products alleging to contain the substance were seized compared to a minuscule fraction of that (26) in 2020.
Botulism is a serious paralytic illness caused by botulinum neurotoxin (BoNT), mainly produced by the bacterium clostridium botulinum. The disease naturally occurs in four different forms:
Food-borne botulism occurs when spores of the organism clostridium botulinum have germinated, and the bacteria have reproduced outside the body and produced toxin.
This occurred in the outbreak in Bordeaux in 2023, during the Rugby World Cup that was linked to home-preserved canned sardines. The outbreak led to the tragic death of a woman and serious illness in others.
Infant botulism is extremely rare but occurs when babies ingest spores which germinate to produce the bacterial cells that reproduce in the gut and release toxin. Honey is a recognised cause of infant botulism.
Wound botulism has the same symptoms as the other forms but occurs when the organisms get into an open wound and are able to reproduce. Cases have been known to occur in intravenous drug users.
The recent health scare is due to iatrogenic botulism that can occur as an adverse event following the administration of BoNTs for cosmetic or therapeutic reasons.
While it is considered rare, individuals receiving BoNT injections for cosmetic purposes (including for facial wrinkle lines) or therapeutic treatments such as management of muscle spasticity may develop botulism if they are injected with an excessive dose of BoNTs.
Symptoms of iatrogenic botulism include weakness and fatigue. Toxicities following cosmetic treatment can include blurred vision, drooping eyelids, difficulty in swallowing, and dry mouth.
The symptoms of botulism can be very severe and require intensive care treatment as well as the administration of botulinum anti-toxin. Even when such treatments are available, complete recovery usually takes weeks to months.
The recent outbreak of iatrogenic botulism in the UK is not the first. The European Centre for Disease Prevention and Control (ECDC) gave an update on iatrogenic botulism cases in Europe in 2023 following 87 cases of botulism linked to and intragastric injection of BoNT reported in Germany (30), Austria (1), France (1), Switzerland (2) and Turkey (53).
The indications were that all cases had the medical interventions aimed at helping them lose weight. The cases were reported to have received the BoNT injections at two private hospitals in Turkey.
Symptoms ranged from mild to severe, with several patients hospitalised. A number were reported to be admitted to intensive care and received treatment with botulinum antitoxin.
Investigations by Turkish authorities revealed licenced BoNT products were administered off-label for the treatment of obesity by intragastric injections. The relevant departments of both hospitals had their activities suspended, pending the results of investigations launched against the parties involved.
Botox cosmetic treatments are costly in registered clinics and in the hands of clinical professionals.
But risking your life by allowing an unregistered individual to inject an unlicensed product, that is one of the most lethal substances known to man, into your skin is reckless.
The recent outbreak in the UK, that resulted in dozens of cases of botulism, demonstrates just how perilous unlicensed products are.
After the outbreak of iatrogenic botulism in the UK, the Department of Health and Social Care released a statement on August 6, announcing new measures to crackdown on unsafe cosmetic procedures will be introduced by the government to protect the public. This includes clinics administering fillers and Botox, who will need to meet strict standards to obtain a licence.
If you want to avail of Botox, the bottom line is to make sure it is a product that is sold or prescribed in accordance with a prescription given by an appropriate practitioner, such as a doctor or other qualified healthcare professional. Otherwise, you are taking a chance with a potentially lethal toxin.
Is that a chance you are prepared to take for the sake of a few less wrinkles or facial lines?
Dr Catherine Conlon is a public health doctor in Cork







 App?
App?


